
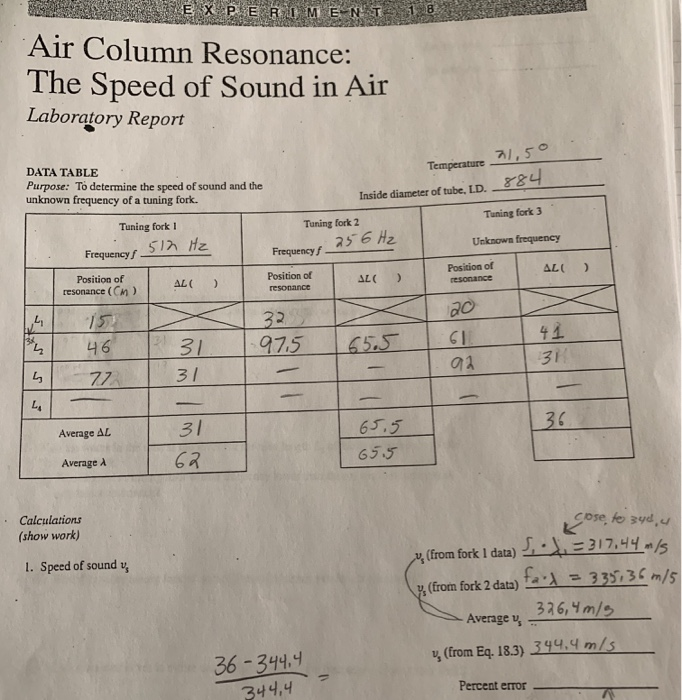
- Lab report assistant data table 3. determination of unknown manuals#
- Lab report assistant data table 3. determination of unknown code#
- Lab report assistant data table 3. determination of unknown plus#
Are cervical dysplasia, CIN III, and severe dysplasia of the cervix reportable?ĬIN III and carcinoma of the cervix in situ are no longer reportable to NPCR or CoC and are not reportable for SEER starting with cases diagnosed after Ĩ. PUNLMPs are pre-malignant growths in the upper urinary tract (renal pelvis, ureters, urinary bladder, part of the urethra).ħ. Are bladder papillary urothelial neoplasms of low malignant potential (PUNLMPs) reportable? NAACCR Layout Version 12: Comparison of Reportable Cancers: CoC, SEER, NPCR and CCCR in Chapter III of NAACCR Vol II, Fifteenth Edition.Ħ. Note: The metastases may be found at the same time as the primary carcinoid of the appendix or may occur years later. When there are discontinuous malignant metastasis from the appendix carcinoid.When a regional lymph node or nodes are positive for malignant metastatic carcinoid.
Lab report assistant data table 3. determination of unknown code#
As of, the ICD-O-3behavior code changed from /1 to /3. When are carcinoids of the appendix reportable?Ĭarcinoid, NOS of the appendix is reportable.
Lab report assistant data table 3. determination of unknown manuals#
See the list of synonyms for in situ in the SEER and the FORDS manuals under the data item Behavior.Ĥ. The foci of intraepithelial carcinoma make this reportable. Is ovarian mucinous borderline tumor with foci of multifocal intraepithelial carcinoma reportable? Reportability depends on the primary site: When they originate in the intracranial (intradural) or intraspinal space they are reportable.ģ. “Likely” is not on the list so the case is not reportable. Ambiguous terminology: How is “likely” interpreted?įollow the ambiguous terminology list strictly as written. PCR testing is a service performed pursuant to an agreement with Roche Molecular Systems, Inc.1. When this happens, we will inform you as quickly as possible with the most complete information available. Note: There may be times when weather or operational considerations cause delays in providing test results. ‡Cytology priority requests submitted on Friday are reported on Monday. Specimens submitted for routine tests using a Saturday courier service will receive next-day results. Specimens submitted for routine tests on Saturday using the IDEXX-Direct service will have results delivered on Monday. For IDEXX-Direct service (FedEx), times are central time. Results delivery is based on working days (Monday–Friday). †All times are local to the submitting practice. Comparative evaluation of two in-clinic assays for vector-borne disease testing in dogs. Liu J, Drexel J, Andrews B, Eberts M, Breitschwerdt E, Chandrashekar R.
Lab report assistant data table 3. determination of unknown plus#
Performance of SNAP 4Dx Plus and AccuPlex4 in dogs with different heartworm burdens.

Performance comparison of SNAP 4Dx Plus and AccuPlex4 for the detection of antibodies to Borrelia burgdorferi and Anaplasma phagocytophilum.



 0 kommentar(er)
0 kommentar(er)
